Song Lab
Welcome to the Song Lab website, where you’ll find an overview of our research group, key focus areas, recent publications, available positions, and contact details. Our lab specializes in cancer epidemiology, with a focus on the intersection of nutrition, the microbiome, and clinical translation.
Kresge 802
677 Huntington Avenue
Boston, MA 02115
Welcome to the Song Lab
The Song Lab is under the charge of the Principal Investigator — Dr. Mingyang Song.

Song Lab Summary
The lab focuses on the clinical and translational epidemiology of cancer. One aspect of the work is to integrate large-scale observational studies with biomarker-based randomized clinical trials to identify novel nutritional and gut microbiota-targeted strategies for cancer prevention and treatment. Another part of the work involves integrating electronic health record (EHR) data with molecular profiling to develop cost-effective risk assessment tools for precision cancer screening and surveillance.
Dr. Song was awarded the NextGen Star by the American Association for Cancer Research. The National Cancer Institute and American Cancer Society support Dr. Song’s current research. The ultimate goal of this research is to translate epidemiologic advances into the clinic for improved cancer prevention and treatment
Song Lab is actively seeking postdoctoral research fellows and students who are interested in the clinical and translational epidemiology of cancer. Please email Dr. Song (msong@hsph.harvard.edu) if you are interested in joining us!
Our Research
Over the past few years, our group has studied the role of diet and lifestyle factors, in conjunction with host immune factors and the gut microbiota, in colorectal cancer development and survivorship. Much of the work is based on three large prospective cohort studies, the Nurses’ Health Study I and II, and the Health Professionals Follow-up Study, in which diet, lifestyle and colorectal cancer diagnosis and mortality have been assessed over decades with blood, stool, and tumor tissue specimens collected in a subset of participants. Building on the findings from observational studies, we are conducting two biomarker-based clinical trials of omega-3 fatty acid treatment in colon cancer patients (supported by the American Cancer Society) and individuals with a history of colorectal adenoma resection (supported by the National Cancer Institute) at Massachusetts General Hospital to investigate causality and explore the potential for future clinical translation. Our group is also involved in the Microbiome among Nurses Study (MICRO-N), which represents the world’s largest prospective collection of microbiome specimens from 25,000 individuals. Leveraging the integrated longitudinal data, we are studying the interplay between diet/lifestyle and gut microbiome in colorectal cancer.

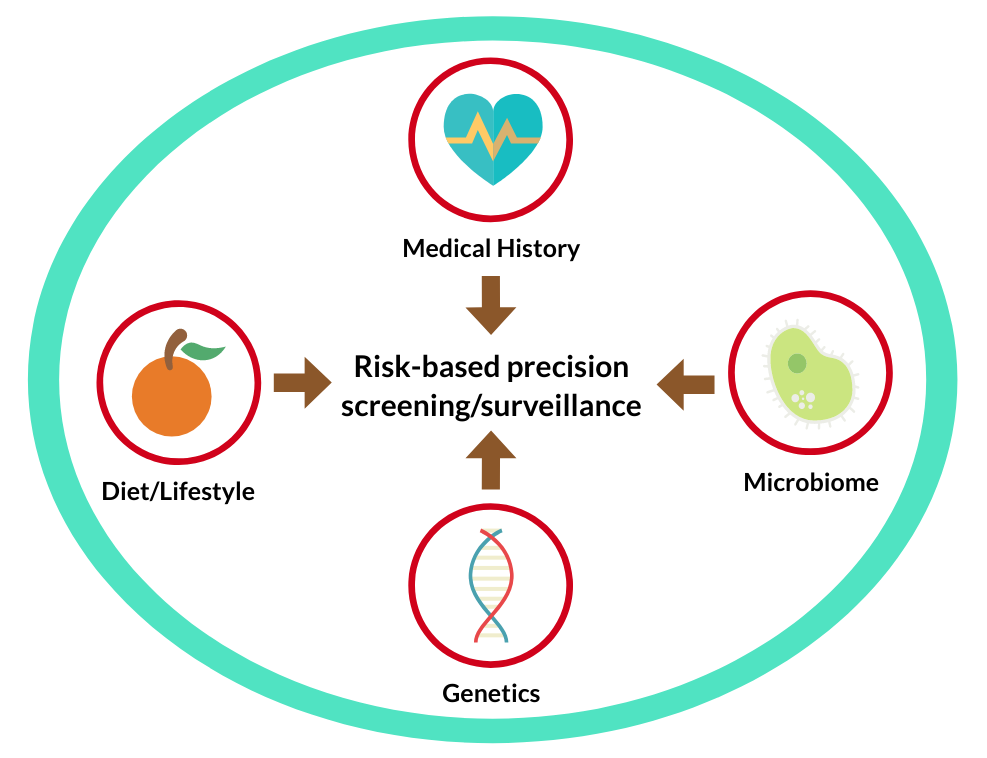
While substantial advances have been made in epidemiology to identify environmental and genetic risk factors, this knowledge has not yet been effectively translated into the clinic for better patient care. A particular example is the age-based or “one-size-fits-all” colonoscopy screening approach that does not take into account the individual variation in colorectal cancer risk. Similar issue occurs to colonoscopy surveillance after polypectomy, where histopathologic features of polyps that are being used in clinical guidelines demonstrate poor sensitivity in predicting subsequent risk of colorectal neoplasia. As a result, lower-risk patients can undergo unnecessary excess testing, whereas higher-risk patients may receive delayed or no testing. To address these gaps, our group is building a longitudinal cohort of patients who had undergone repeated colonoscopy exams in the Partners HealthCare. Detailed clinical and epidemiologic data are being extracted from the EHR systems supplemented by use of validated natural language processing algorithms; and then linked to the state cancer registry for cancer incidence and to the Partners Biobank for genomic information. Tissue specimens will be collected from the pathology departments for tumor profiling. This integrated cohort will allow us to identify novel biomarkers for early detection, validate prediction models in the real clinical setting, and develop and evaluate clinically applicable risk assessment tools for precision screening and surveillance.
Support Harvard Chan School
Every gift contributes to our mission of building a world where everyone can thrive. To learn more about how you can support the Song Lab, please contact Laura Barnes at lbarnes@hsph.harvard.edu.

