Park Lab
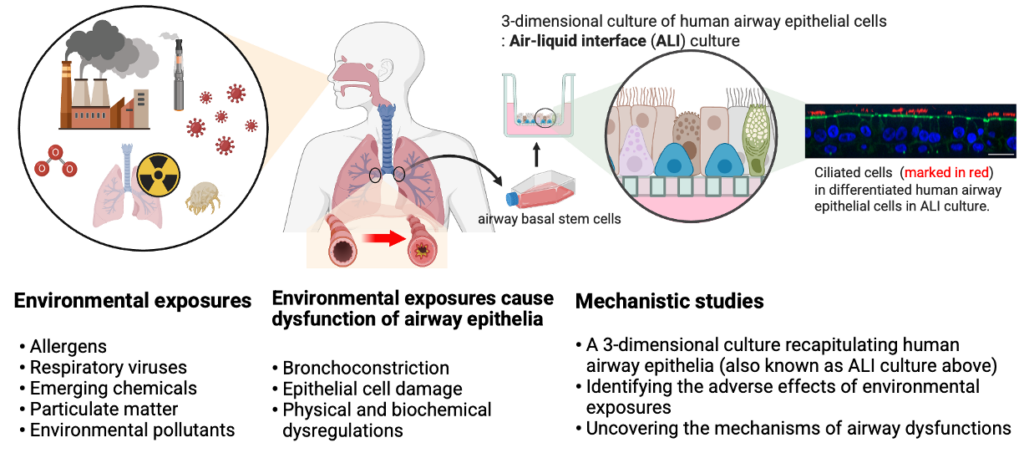
Research at the Park Lab is focused on understanding the role of the airway epithelium in the lung. As the primary barrier against external stimuli—ranging from environmental pollutants and allergens to bacteria and viruses—the airway epithelium serves as the first line of defense for respiratory health.
665 Huntington Avenue
Building 1, Room 306
Boston, MA 02115
About the Lab
Our research interest lies in understanding the role of the airway epithelium in the lung. As the primary barrier against external stimuli—ranging from environmental pollutants and allergens to bacteria and viruses—the airway epithelium serves as the first line of defense for respiratory health. While airway epithelial cells contribute significantly to lung homeostasis under normal conditions, their defense mechanisms are dysregulated under chronic conditions such as chronic obstructive pulmonary disease (COPD) and asthma.
Airway Remodeling
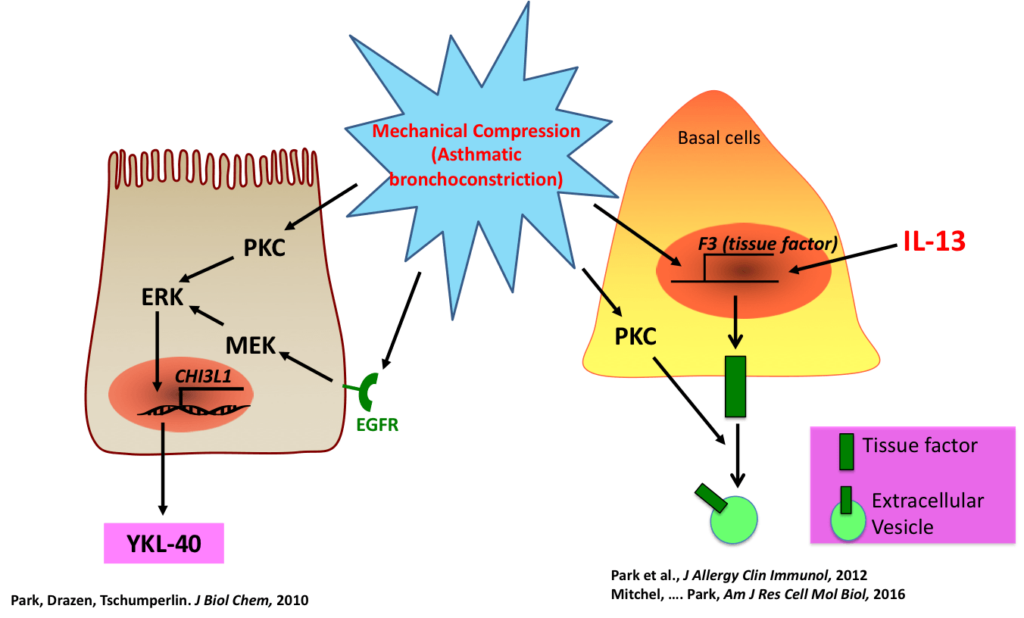
In asthma, a hallmark of disease is airway remodeling, but its cause is largely unknown. Most theories of airway remodeling have attributed the observed changes to the effects of mediators and cytokines derived from inflammatory cells, and little or no attention has been paid to the impact of bronchoconstriction itself. The focus of the research conducted in our lab is to examine the processes by which mechanical compressive stress that is imposed on the airway epithelium, such as the stress that occurs during bronchoconstriction, initiates airway remodeling, even in the absence of inflammatory cells. We consider physical forces as an essential cue in the maintenance or disruption of cellular homeostasis. The goal of our research is to define the physical force-mediated mechanisms in chronic airway disease, particularly in situations where patients frequently experience bronchoconstriction.
Unjamming of airway epithelial cells
Tissue remodeling — including embryonic morphogenesis, angiogenesis, and wound repair — depends on collective cellular migration. Rather than moving individually, cells of the epithelial layer migrate cooperatively in sheets, ducts, strands, and clusters. However, collective migration of epithelial cells has never been studied in the context of airway remodeling in asthma.
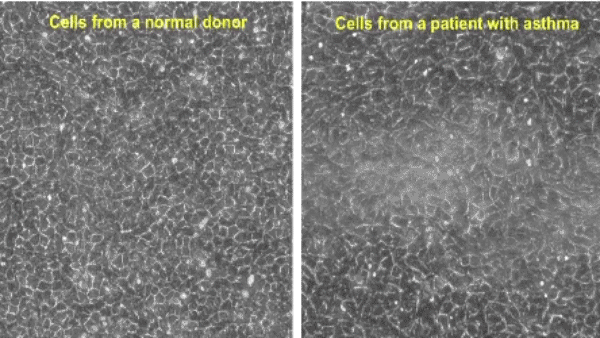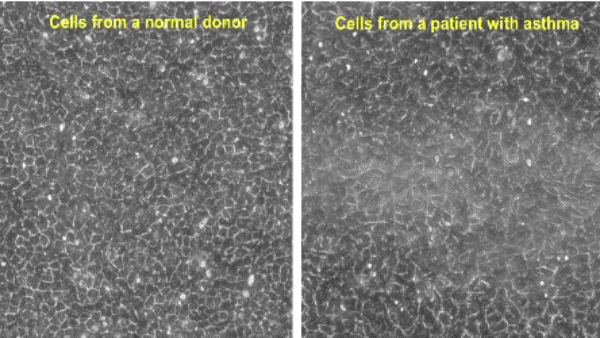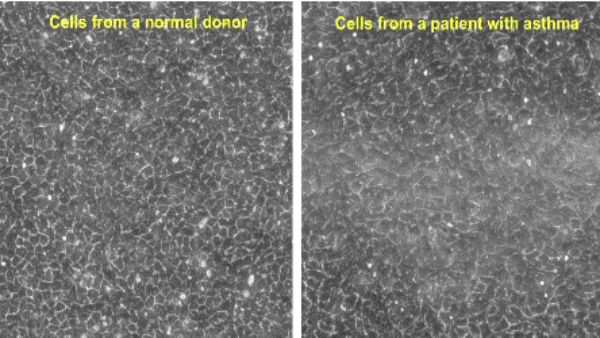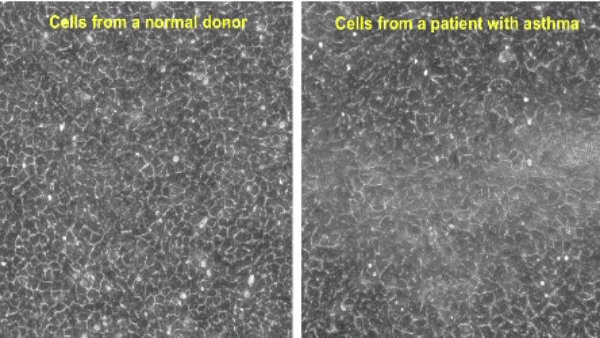
Using well-differentiated human airway epithelial cells maintained in ALI culture (Unjamming and cell shape in the asthmatic airway epithelium; Park et al, Nature Materials, 2015), we identified the collective migration of confluent airway epithelial cells. During maturation, a cellular layer undergoes a phase transition from an unjammed phase, in which cells move like a fluid and rearrange with neighbors frequently, to a jammed phase, in which cells move little like a solid and rearrange with neighbors infrequently. As compared with non-asthmatic cells, this phase transition toward the jammed phase is delayed in asthmatic cells. The perturbation of the jammed layer with compressive mechanical stress reverses the natural and spontaneous phase transition, causing the jammed layer to revert to the unjammed phase, as termed the unjamming transition (UJT). In the same publication, we also reported that cell-shape parameter can be a rigorous tool in determining whether the layers are in a jammed or an unjammed phase.
Since the discovery of the UJT in 2015, the UJT has been considered as an alternative mechanism of cellular migration, in addition to the epithelial-mesenchymal transition (EMT), which has been traditionally recognized as a major mechanism of cellular migration. In a newly published work (Mitchel. et al, Nature Communications, 2020), we have shown that the EMT and the UJT are distinct biological programs, thus providing new insights on a wide range of human diseases and basic biological processes. In well-differentiated HBE cells, the UJT is induced by pathologic stimuli linked to lung disease, including ionizing radiation (O’Sullivan et al., Frontiers in Cell and Developmental Biology, 2020) and mechanical compression (Park et al., Nature Materials, 2015; Mitchel et al., Nature communications, 2020). The goal of our research is to establish a novel biophysical framework for understanding collective cellular migration in lung health and disease.
Role of extracellular vesicles for intercellular communications in asthma
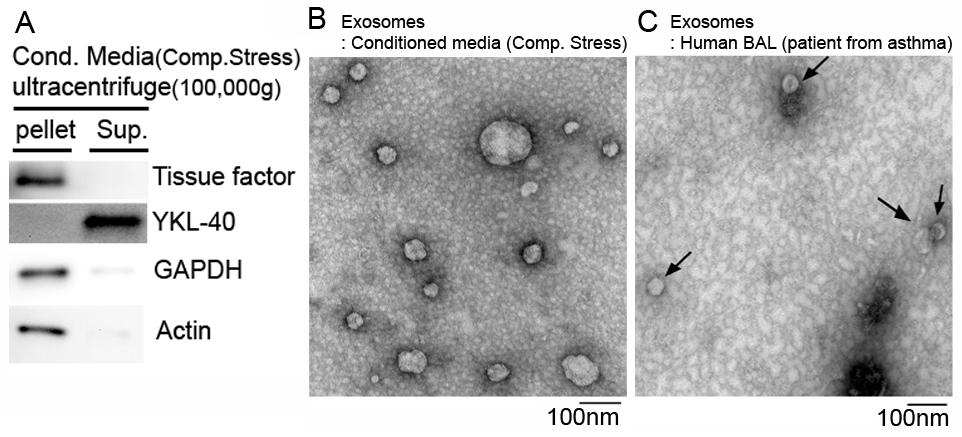
Extracellular vesicles (EVs), including exosomes, microvesicles, microparticles, and apoptotic bodies are increasingly recognized as a major means of cell–cell communication. The size of EVs ranges from 30 nm to 1000 nm in diameter, depending on their origin. The EVs contain lipids, proteins, and genetic materials such as mRNA and miRNA and may serve as both disease markers and novel therapeutic tools. More importantly, EVs can contribute to disease pathogenesis. However, the role of EVs and the mechanisms underlying their function in asthma are unknown. The goal of our research is to determine the role of the EVs released from airway epithelial cells in asthma pathogenesis.
Learn more…
- View our Latest Publications
- View Research Opportunities with the Park Lab