MEMCARE-SRC
The Metals and Metal Mixtures, Cognitive Aging, Remediation and Exposure Sources (MEMCARE) Superfund Research Center aims to understand how metals and metal mixtures contribute to cognitive decline in older age and the biological mechanisms underlying these effects; and to develop new ways to detect and remove metal contaminants in drinking water sources.
677 Huntington Avenue
Boston, MA 02115
Project 2: Brain Mechanisms
Effects of metal exposures on brain extracellular vesicle (EV) microRNA
The mechanisms by which metals can have long term or delayed effects on brain function are not well understood. In the nervous system, and throughout the body, cells communicate with and regulate the activity of nearby cells by releasing extracellular vesicles (EVs) containing signaling molecules like microRNAs (miRNA), which can influence gene expression in target cells.
Project 2 investigates the hypothesis that metal exposures in early life alter EV miRNAs in the brain and that these changes affect the functioning of neural cells resulting in accelerated cognitive aging later in life.
Project Goals
The aim of Project 2 is to determine the functional role of selected EV miRNAs (based on blood samples from the Project 1 EV biomarker studies):
- in cultured neural and neuroglial cells.
- on cognitive function in mice, both alone and in the context of environmental exposures to metal toxicants.
Our Approach
Microglial cells in the brain play an important role in responding to environmental insults including exposure to lead and arsenic. Our project uses cultured microglial cells to study the impact of metal exposures on EV content and function.
In particular, we are examining the effects of exposure to individual metals (lead, arsenic, manganese or cadmium) as well as to “real-world” metal mixtures (from pre- and post-remediation water samples collected by our Community Engagement Core at the San Luis Valley Superfund site) on the secretion of microglial EV miRNAs. Results of these studies will inform and complement additional studies using human plasma EV miRNA from subjects in Project 1.
In addition, we will determine the effects of metal exposures on brain organoid formation and on neural stem cell proliferation and differentiation, as well as on cognitive function in mice.
FAQs
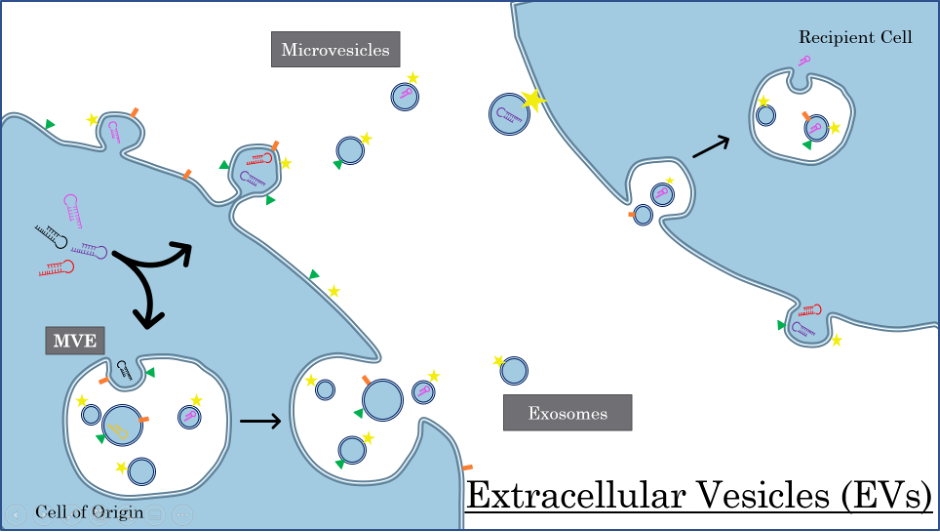
Extracellular vesicles or EVs are tiny packages containing proteins, nucleic acids or other molecules that are released by cells to communicate with nearby cells and tissues. There are many different types and sizes of EVs, including exosomes, microvesicles and apoptotic bodies (not shown), and almost all cells release them.
Microvesicles bud directly from the cell into the extracellular space. Exosomes are formed inside large multivesicular endosomes (MVE) which travel to the cell membrane and expel their contents. The EVs dock onto the surface of a recipient cell where they may remain and/or share their cargo.
MicroRNA (miRNA) is a small (about 22 nucleotides long) molecule of RNA that can regulate gene expression by binding to messenger RNA (mRNA) inside a cell and preventing or delaying use of that mRNA in protein translation. If there is too much or too little of a particular miRNA, the protein that it ultimately regulates will be under or over expressed.
The quantity and content of extracellular vesicles (EVs) released by cells changes in diseases and in response to environmental exposures. To study these changes, scientists can measure EVs in body fluids (like blood, saliva or cerebral spinal fluid), or grow cells and organoids (simplified multi-cell models of a bodily organs, like the brain) in tissue culture dishes in the laboratory and study their EV responses under carefully controlled conditions.
Neuroglia are non-neuron cells in the nervous system that perform specialized “support” functions. Microglia are one type of neuroglia found in the brain, where they are important for repairing and removing damaged neurons and eliminating invading micro-organisms.
Microglia are involved in our nervous system’s response to environmental insults, including exposure to lead and arsenic, and dysfunction of microglia has been shown to play a role in neurodevelopmental and neurodegenerative diseases. In Project 2, we will examine if and how signaling via EVs is important for microglial function, and how metal exposure may affect it.
Project 2 is currently working with EVs from three microglial models:

- We culture and isolate microglial cells extracted from mouse brain.
- We grow microglia from human induced pluripotent stem cells (iPSCs). iPSCs are regular cells that have been genetically reprogramed in the laboratory to have the ability to differentiate into any kind of cell.
- We measure EVs expelled into the culture media by microglia-containing brain organoids grown in the lab of our collaborator, Lotje DeWitte at Mt. Sinai Medical Center. An organoid is a simplified multi-cell model of a bodily organ like the brain, that can be grown in a laboratory for research.
Project Team
Recent Publications
Zunwei Chen, Qiyu Wang, Quan Lu, Engineering ARMMs for improved intracellular delivery of CRISPR-Cas9, 2025, Extracellular Vesicle, Volume 5,100082
Park HR, Azzara D, Cohen ED, Boomhower SR, Diwadkar AR, Himes BE, O’Reilly MA, Lu Q. Identification of novel NRF2-dependent genes as regulators of lead and arsenic toxicity in neural progenitor cells. J Hazard Mater. 2024 Feb 5;463:132906.
Zunwei Chen, Zhi Qiao, Charlotte R Wirth, Hae-Ryung Park, and Quan Lu. 12/2023. “Arrestin domain-containing protein 1-mediated microvesicles (ARMMs) protect against cadmium-induced neurotoxicity.” Extracellular Vesicle, 2, Pp. 100027.
Sengjin Choi, Zhiping Yang, Qiyu Wang, Zhi Qiao, Maoyun Sun, Joshua Wiggins, Shi-Hua Xiang, and Quan Lu. 2023. “Displaying and delivering viral membrane antigens via WW domain-activated extracellular vesicles.” Sci Adv, 9, 4, Pp. eade2708.