In liver, a stressed cell can be bad news for its neighbors
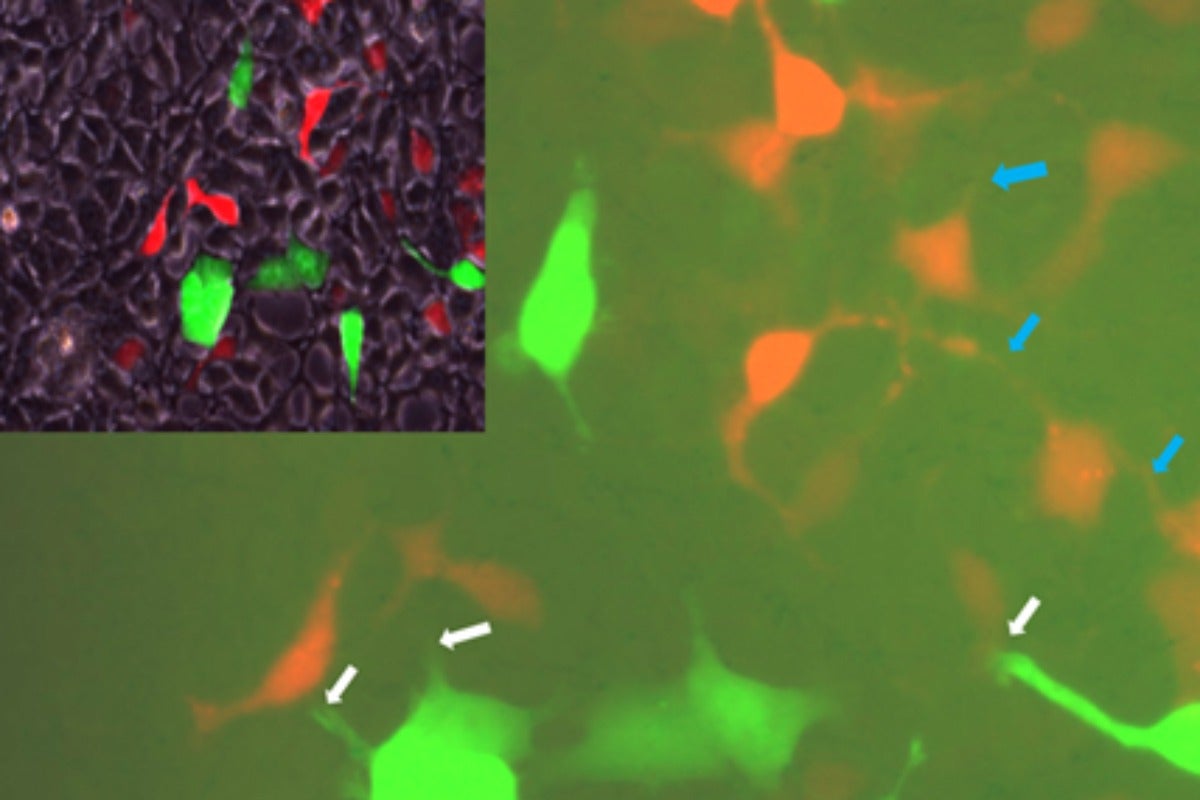
Findings provide new insights into obesity, diabetes, and fatty liver disease
For immediate release: December 18, 2020
Boston, MA – A key protein in the communication channels between cells can allow a stress response in one liver cell to spread to neighboring liver cells in mice, causing otherwise healthy cells to become dysfunctional, according to new research co-led by Harvard T.H. Chan School of Public Health and Sheba Medical Center in Israel. The findings could have implications for a range of metabolic diseases, including obesity, diabetes, and non-alcoholic fatty liver disease (NAFLD).
The study is the first to demonstrate that the protein, Cx43, plays a role in the spread of endoplasmic reticulum (ER) stress signals among liver cells. Remarkably, the researchers noted, mice lacking Cx43 in their livers were protected from insulin resistance, glucose intolerance, and NAFLD.
The findings were published in Cell Metabolism on December 18, 2020.
“We found that when a stressed liver cell starts communicating with its neighbors, it can send stress signals to neighboring cells, causing significant problems that can lead to fatty liver disease and metabolic disease,” said corresponding author Gökhan Hotamışlıgil, James Stevens Simmons Professor of Genetics and Metabolism at Harvard Chan School and director of the Sabri Ülker Center for Metabolic Research. “Here, we developed complex methods to follow molecular stress signals as they pass from one cell to another and, importantly, we showed that when these signals are stopped in the originating cell, metabolic health can be preserved even under adverse conditions such as obesity.”
Previous research in the Sabri Ülker Center has shown that in obese animals and humans, liver tissue experiences stress and dysfunction of the ER. It has, however, been challenging to understand why this powerful tissue failed to launch countermeasures to mitigate this problem. The researchers found in this study that once a small group of cells in the liver experience stress, it quickly spreads to the rest of the tissue, from cell to cell, overwhelming the tissue’s natural defenses.
For this new study, the researchers first screened billions of cells to find a few that naturally exhibit ER stress and others that have no sign of stress whatsoever. When they were grown next to each other, the stressed cells passed stress signals on to the healthy cells. The team then focused on liver cells isolated from mice. They experimentally induced ER stress in these cells and observed that levels and activity of Cx43 increased. As Cx43 activity increased, these cells became more capable of transmitting stress signals to nearby cells when compared with cells that were not under ER stress.
To build on these initial findings, the research team conducted a series of experiments on mice and determined that diet-induced obesity resulted in ER stress that in turn increased levels and activity of Cx43. The team then created a line of mice in which Cx43 had been deleted from the animals’ liver cells. In these mice, a high-fat diet did not trigger ER stress in liver cells, and the animals were protected from insulin resistance, glucose intolerance, and NAFLD.
“Rates of obesity are rising globally, and we do not fully understand the complications associated with it, including metabolic disease and non-alcoholic fatty liver disease,” said Amir Tirosh, corresponding author and director of the Division of Endocrinology, Diabetes and Metabolism at Sheba Medical Center. “This study shows that cell-to-cell communication plays an important role in spreading stress signals, and it indicates that stopping the transmissions of these stress signals could be an attractive approach to preventing and treating insulin resistance and NAFLD.”
Funding for this study came from National Institute of Health/National Institute of Diabetes, Digestive and Kidney Diseases Career Development Award K08 DK097145-01, the Israel Science Foundation grant No. 922/17, and NIH T32 Training Grant T32DK007529.
Other Harvard Chan School researchers who contributed to the study include Gurol Tuncman, Ediz Calay, Abdullah Yalcin, Yankun Lee, and Ana Paula Arruda.
“Inter-cellular transmission of hepatic ER stress in obesity disrupts systemic metabolism,” Amir Tirosh, Gurol Tuncman, Ediz S. Calay, Moran Rathaus, Idit Ron, Amit Tirosh, Abdullah Yalcin, Yankun G. Lee, Rinat Livne, Sophie Ron, Neri Minsky, Ana Paula Arruda, and Gökhan S. Hotamisligil, Cell Metabolism, December 18, 2020, doi: 10.1016/j.cmet.2020.11.009
Photo: Courtesy of Sabri Ülker Center for Metabolic Research
Visit the Harvard Chan School website for the latest news, press releases, and multimedia offerings.
For more information:
Chris Sweeney
617.432.8416
csweeney@hsph.harvard.edu
###
Harvard T.H. Chan School of Public Health brings together dedicated experts from many disciplines to educate new generations of global health leaders and produce powerful ideas that improve the lives and health of people everywhere. As a community of leading scientists, educators, and students, we work together to take innovative ideas from the laboratory to people’s lives—not only making scientific breakthroughs, but also working to change individual behaviors, public policies, and health care practices. Each year, more than 400 faculty members at Harvard Chan School teach 1,000-plus full-time students from around the world and train thousands more through online and executive education courses. Founded in 1913 as the Harvard-


