AKT-mediated phosphorylation of TSC2 controls stimulus- and tissue-specific mTORC1 signaling and organ growth
Growth factor signaling pathways are known to drive tissue growth and development, but how these molecular mechanisms function across different organs in a living organism has remained unclear. In particular, the physiological role of AKT-mediated activation of mTORC1—a central regulator of nutrient and growth signaling—has yet to be defined in vivo.
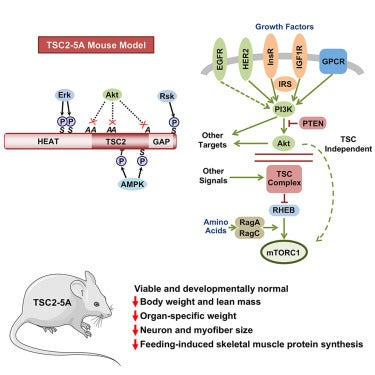
In a new study published in Developmental Cell, the Manning lab developed a mouse model (TSC2-5A) in which five critical AKT phosphorylation sites on the TSC2 protein are mutated. This phospho-mutant TSC2-5A mouse uncouples AKT from the TSC complex and mTORC1 activation, allowing direct investigation of this signaling step in a physiological context.
Despite disrupting a major input to mTORC1, TSC2-5A mice are viable and develop normally, in contrast to the embryonic lethality observed with complete loss of TSC components. However, these mice exhibit an overall reduced body weight and selective deficits in organ growth. Notably, TSC2-5A mice display smaller brains, with neuron-intrinsic decrease in cell size and mTORC1 activity. The mice also have decreased skeletal muscle mass and show impaired muscle protein synthesis after feeding.
These findings demonstrate that AKT-mediated phosphorylation of TSC2 is a critical mechanism for promoting mTORC1 activity in select tissues. Moreover, the TSC2-5A model offers a powerful genetic tool to study how mTORC1 integrates hormonal and nutrient signals to coordinate growth, with implications for understanding developmental biology, metabolic homeostasis, and aging.