Food Allergy News & Updates

In this issue, I highlight our ongoing research on food allergies and the dedicated scientists in my lab who are advancing this field during these challenging times. Advancing science requires curiosity, patience, and resilience—to continuously confirm, build upon, or challenge prior findings. For more than 30 years, our work has focused on preventing, diagnosing, and treating food allergies, and over time, our efforts have led to meaningful clinical progress. We prioritize collaborations and continue to work with Stanford, Univ of Chicago, Northwestern, FARE, London, Zurich, Munich, Denver, Cincinnati, and others.
Here are some of the clinical trials that are underway focused on preventing and treating food allergy. There is growing evidence that food allergy begins very early in life. This is the focus of our SEAL and Project Viva studies. We are also evaluating new approaches to treating food allergy. Although oral immunotherapy (OIT) has been shown to be effective, several barriers remain, including allergic reactions during treatment, lengthy treatment duration, and the need for frequent clinic visits. In addition, while most patients achieve desensitization, this effect is often temporary and may diminish when the allergenic food is no longer consumed on a regular basis. To address these challenges, we are exploring the use of biologics (with or without OIT) to improve safety, enhance efficacy, and achieve lasting tolerance.
Thank you for your continued interest and support in the work we do in Food Allergy.
Best,
Principal Investigator, Allergy, Extreme Weather, & Exposomics Lab
John Rock Professor of Climate and Population Studies
Chair, Department of Environmental Health

In this issue:
Research Updates
SEAL (Stopping Eczema and Allergy Study)

What causes some individuals to become allergic while others remain tolerant? Part of the answer lies in our genes. For example, those with a mutation in the gene, filaggrin, which codes for the skin barrier protein, are at much higher risk of developing allergies. Interestingly, they are not just at risk of developing eczema which is an allergic skin disorder, they are also at risk of developing other allergies diseases such as food allergies, allergic asthma, or allergic rhinitis.
Another interesting observation is that most individuals with one allergy are at greater risk of developing other allergies, and that there appears to be a sequential order by which these allergies develop. For example, eczema is often the first allergy to develop, often in early infancy generally followed by food allergy, allergic rhinitis, and allergic asthma. This sequence of manifestation of allergic diseases has been termed the Atopic March (see Figure 1 below)
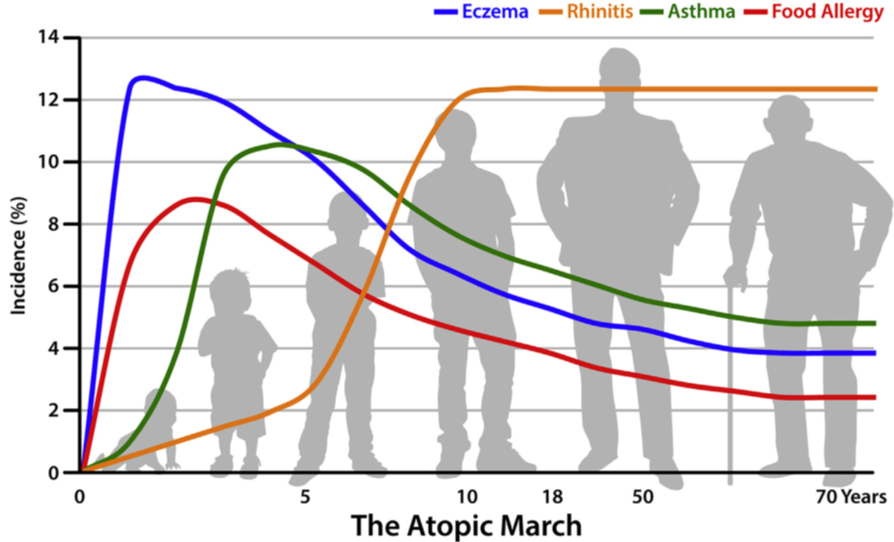
These observations led to the hypothesis that allergic sensitization may occur when inflamed and/or disrupted skin are exposed to environmental allergens and that the different allergies are varying manifestations of the same disease in different organ systems with a common underlying mechanism. Preliminary evidence suggests that by improving skin barrier integrity we may potentially reduce eczema and other allergies.
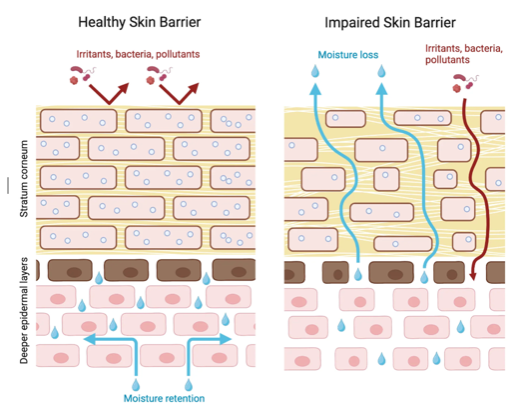
We are therefore conducting a large multicentered 2-year study called SEAL (Stopping Eczema and ALlergy study) to determine whether early intervention with skin moisturizing agent with a trilipid moisturizer in a high-risk infant group with eczema or dry skin can reduce the severity of eczema and decrease risk of developing food allergy. A trilipid moisturizer is one that mimics the skin natural lipid composition as well as physiological pH. These infants will be compared to two control groups of infants, one with no intervention and the other receiving a standard (non trilipid) moisturizer. The study is being conducted at four centers in the USA and one center in the UK. We have currently recruited 398 infants, of whom 397, 241, and 139 have completed baseline, 12 month, and 24 month visits, respectively. Learn more about the study here.
Project Viva
Does a maternal diet influence risk of food allergy? Project Viva is a ground breaking longitudinal research study of mother-child pairs that was started in 1999 to find ways to improve the health of mothers and their children. The study examines the effects of mother’s diet, as well as other factors during and after pregnancy, on child health outcomes, including food allergy.

For example, the information collected enables us to investigate the effects of diet on child development and obesity, and how diet and the environment influence the development of asthma and allergies in children.
Comprehensive dietary and environmental assessments in pregnancy, prospective follow-up, and well-characterized allergic outcomes are being conducted. Prior analyses using this cohort have demonstrated robust associations of prenatal diet with growth, obesity, and asthma, underscoring feasibility.
A total of 2128 babies born between 1999 and 2003 have been enrolled into Project Viva since 1999, and many are still actively engaged with the study. Learn more about the study here.
OUtMATCH
The Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen OIT in Food Allergic Participants (OUtMATCH) study is a clinical trial that was conducted across 10 centers in the United States. The clinical phase of the trial has been completed, and data are continuing to being analyzed.
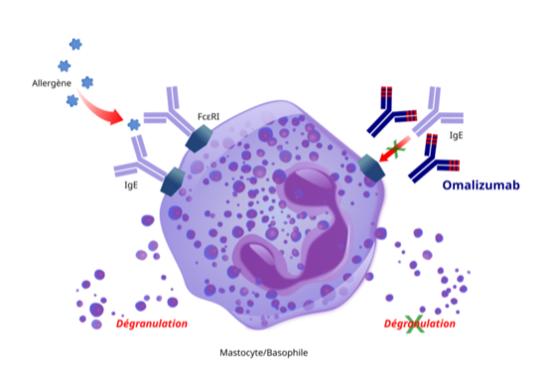
Omalizumab has been approved for asthma, chronic urticaria, chronic rhinosinusitis with nasal polyps, and for food allergy. For patients with IgE-mediated food allergies, treatment is designed to reduce the risk of harmful allergic reactions. It does not eliminate food allergies or allow patients to consume food allergens freely. However, its repeated use will help reduce the health impact if accidental exposure occurs.
Omalizumab targets a key antibody, IgE, that is common in many allergic diseases. In individuals who are sensitized to allergens, IgE can be found attached to a receptor on certain immune cells (mast cells or basophils). When an individual encounters an allergen, the allergens trigger IgE on mast cells to release inflammatory molecules such as histamine. Omalizumab can preempt the release of histamine by blocking the ability of the allergens to trigger histamine release.
Oral immunotherapy (OIT) has been used successfully for desensitizing individuals with food allergy so that individuals do not react to allergens. However, it is time consuming, labor intensive, often requiring many trips to the allergy clinic. Palforzia, a standardized peanut protein powder has been approved for use in patients with peanut allergy. However, for those with other food allergies there are currently no FDA approved products. Additionally, OIT is not a cure as individuals often become allergic again after discontinuation of treatment. Its main mode of action is by upregulating IgG4, a molecule that blocks IgE. With prolonged treatment, IgE also decreases. Decreases in a number of proinflammatory cells and molecules are also observed with treatment.

This study follows up previous successes with omalizumab and oral immunotherapy in those with multiple food allergies. The study was designed to answer the following three main questions:
- Does taking omalizumab for a certain length of time stop or decrease allergic reactions to peanut and other common food allergens?
- How does a short course of omalizumab combined with multi-allergen oral immunotherapy compare with a longer course of omalizumab in decreasing allergic reactions?
- After participants stop both treatments, are they able to eat peanut and the 2 other foods in the form that is normally eaten?
A total of 177 adults and children were enrolled in the study. The study found that omalizumab treatment for 16 weeks was superior to placebo in increasing the amount of peanut and other common food allergens that individuals could consume. Molecular changes associated with desensitization was observed in the omalizumab group but not in the placebo group. These results have been published. Other analyses and publications are in progress. Read more about the study here.
COMBINE
COMBINE is a clinical trial that is evaluating the safety and efficacy of using 2 biologics (dupilumab and omalizumab) for the treatment of food allergy.
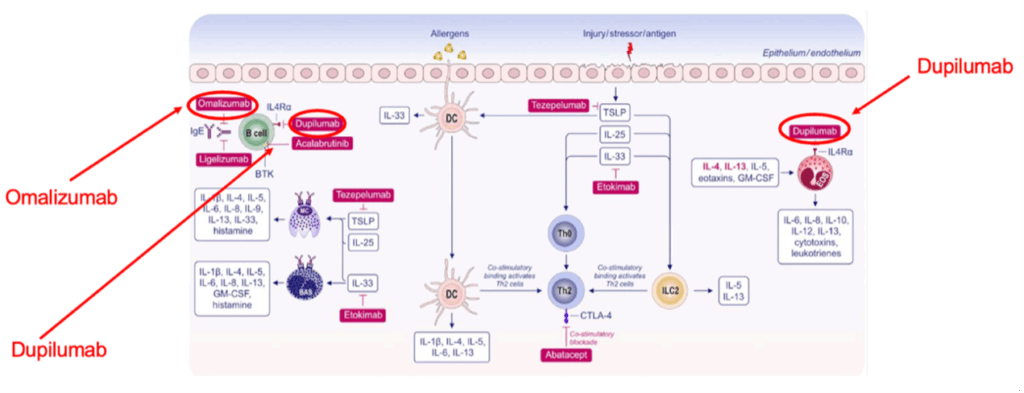
While omalizumab blocks IgE directly, dupilumab reduces allergic reaction by blocking IgE production by B cells (see figure 4 above). Omalizumab has been approved for asthma, chronic urticaria, chronic rhinosinusitis with nasal polyps, and for food allergy. Dupilumab has been approved for treatment of eczema, asthma, chronic rhinosinusitis with nasal polyps, and other diseases.
In COMBINE, we hypothesized that treating multi-food allergic patients with omalizumab and dupilumab together will lead to a better desensitization to food allergens than using only omalizumab or only dupilumab or placebo.
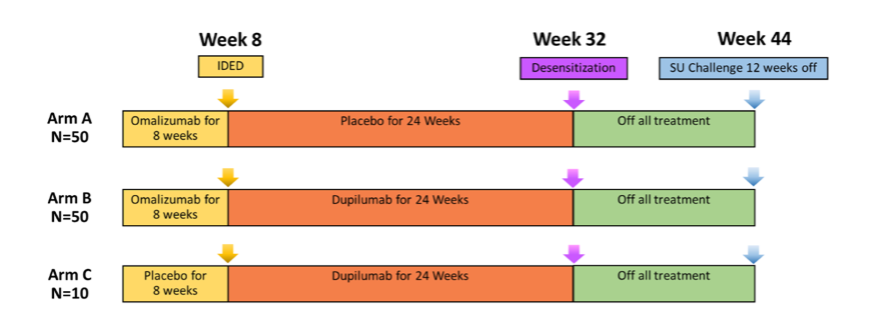
Around 110 participants, ages 4 to 55 years with a history of multiple food allergies have completed the study. Participants were enrolled in one of 3 study arms and the study design is shown in Figure 5, above. Data are currently being analyzed. Learn more about the study here.
Meet the Scientists
Carmela Pablo-Torres Jimenez, PhD
I grew up in Spain. As an infant, I developed eczema (also called atopic dermatitis). Soon after, I also developed allergies to pollen (grasses and olive), house dust mites, and animal dander, which triggered allergic rhinitis and asthma. It significantly affected my quality of life and that of my family, as the itching was painful, my sleep was disrupted, and I often required emergency visits due to respiratory difficulties. I always wondered why I, but not many others, were allergic to pollen and other allergens.
So, when I took an immunology class in college, I was fascinated to learn about immune cells and how they protect the body. I also learnt how they sometimes become hyperactive and attack innocuous substances causing allergic reactions or even attack one’s own’s cells causing autoimmune diseases. My professors Dr. Diaz-Perales and Dr. Blanco were passionate about immunology, and their passion was contagious. I knew then that I wanted a career in immunology.
A few years later, I obtained a PhD in immunology in the research group of Dr. Barber and Dr. Escribese at San Pablo CEU University in Madrid. During my training, I attended Dr. Nadeau lectures at conferences on allergies and asthma and found them fascinating. I applied for a postdoctoral position in her lab at Harvard and was thrilled when I got the position. I have worked in her lab for 1 year now and am constantly learning and discovering.
I am a Senior Research Scientist in Dr. Kari Nadeau’s laboratory in the Department of Environmental Health at the T.H. Chan School of Public Health. I received my Bachelor of Medicine and PhD from the Xiangya School of Medicine, Central South University (China), followed by postdoctoral training at the Keck School of Medicine, University of Southern California.

Xiaoying Zhou, PhD

I am excited to be a part of Dr. Nadeau’s research team. I am currently working on COMBINE and OUtMATCH food allergy clinical trials, where we are investigating biological drugs and immunotherapy for safe and effective treatments for food allergies. I do that by examining how proteins on immune cells are expressed or altered in allergy and how they are modified with treatment. Understanding these changes can enable us to develop treatments to target key molecules in the allergic pathway.
I enjoy working on food allergy research in Dr. Nadeau’s lab. Dr, Nadeau’s lab has made much progress in treating patients with food allergy and I am excited to understand the molecular mechanisms that are associated with allergic diseases and with desensitization. However, there is still so much that is unknown about immunology and allergic diseases. It is like working on a complex puzzle with many dimensions. I am now working on understanding the mechanisms underlying omalizumab, which has been approved for asthma, food allergies, and other diseases. The drug allows people to consume a higher dose of the food allergen that they are allergic to than they were able to previously. We know it blocks a molecule called IgE, a molecule key to releasing histamine and other inflammatory molecules. We are trying to understand other effects of omalizumab and how it may be used with immunotherapy to make food allergy treatment safer and more effective. I also patented a new target for food allergies with Kari and we will let you know more later.
Yagiz Pat, MD
I am a physician–scientist (MD) trained in medical microbiology and immunology. I study the interactions between the microbiome and allergens. In particular, I use a number of high throughput omic technologies to analyze DNA, RNA, proteins, and metabolites. I am also using organoids and organ-on-a-chip systems, which are three-dimensional cell culture models that aim to replicate the structure and function of human organs. These together with other molecular biology techniques can assist us in understanding inflammatory pathways.

The intestinal organoid culture that we use in the Nadeau lab is a state-of-the-art technique which has a high degree of similarity to the human gastrointestinal system. It allows us to study cell responses to environmental factors, including allergens. With this technique, we are planning to investigate how allergens affect the gut barrier and cause inflammation in a patient with food allergies.
Colin Skeen, MS

My name is Colin Skeen, and I am a Research Assistant in Dr. Kari Nadeau’s lab. I am a biomedical engineer with a B.S. from the University of Maryland, College Park and a M.S. from Boston University. As someone with a brother living with severe food allergies, I am excited to join Dr. Nadeau’s lab and work on cutting-edge food allergies research.
I have been working on the Stopping Eczema and Allergy (SEAL) trial, a very exciting Phase II clinical trial. My role is to process participant blood samples, distribute study materials to the clinical sites, and ensure sample collection occurs efficiently and stored correctly so that they can be analyzed. The opportunity to work on a study that aims to prevent food allergies is very exciting!
Recent Publications
- Jiang SY, Cao S, Martinez K, Sharma R, Raeber O, Fernandes A, Bogetic D, Kaushik A, Gupta S, Manohar M, Maeker HT, Chin AR, Long AJ, Feight C, Woch M, Nadeau KC, Chinthrajah RS, Sindher SB. Shrimp oral immunotherapy outcomes in the phase 2 clinical trial: MOTIF. Front Allergy. 2025 Jul 22;6:1458131.
- Zeyneloglu C, Babayev H, Ogulur I, Ardicli S, Pat Y, Yazici D, Zhao B, Chang L, Liu X, D’Avino P, Li M, Biçer C, Kurtoğlu Babayev FH, Dhir R, Nadeau KC, Brüggen MC, Akdis M, Akdis CA. The epithelial barrier theory proposes a comprehensive explanation for the origins of allergic and other chronic noncommunicable diseases. FEBS Lett. 2025 Jul 18.
- Castaño N, Chua K, Nguyen A, Marshall W, Hofmann GH, Lee J, Tsai M, Sindher SB, Nadeau KC, Chinthrajah RS, Galli SJ, Tang SKY. A hand-operated microfluidic sample preparation-to-analysis workflow for simplifying the basophil activation test. Lab Chip. 2025 Jul 23;25(15):3779-3791.
- Hund SK, Sampath V, Zhou X, Thai B, Desai K, Nadeau KC. Scientific developments in understanding food allergy prevention, diagnosis, and treatment. Front Immunol. 2025 Apr 22;16:1572283.
- Chinthrajah RS, Sindher SB, Nadeau KC, Leflein JG, Spergel JM, Petroni DH, Jones SM, Casale TB, Wang J, Carr WW, Shreffler WG, Wood RA, Wambre E, Liu J, Akinlade B, Atanasio A, Orengo JM, Hamilton JD, Kamal MA, Hooper AT, Patel K, Laws E, Mannent LP, Adelman DC, Ratnayake A, Radin AR. Dupilumab as an Adjunct to Oral Immunotherapy in Pediatric Patients With Peanut Allergy. Allergy. 2025 Mar;80(3):827-842.
- Zhou X, Dunham D, Sindher SB, Long A, Fernandes A, Chang I, Assa’ad A, Pongracic J, Spergel JM, Tam J, Tilles S, Wang J, Boyd SD, Chinthrajah RS, Nadeau KC. HLA-DR+ regulatory T cells and IL-10 are associated with success or failure of desensitization outcomes. Allergy. 2025 Mar;80(3):762-774.
- Stephen-Victor E, Kuziel GA, Martinez-Blanco M, Jugder BE, Benamar M, Wang Z, Chen Q, Lozano GL, Abdel-Gadir A, Cui Y, Fong J, Saint-Denis E, Chang I, Nadeau KC, Phipatanakul W, Zhang A, Farraj FA, Holder-Niles F, Zeve D, Breault DT, Schmitz-Abe K, Rachid R, Crestani E, Rakoff-Nahoum S, Chatila TA. RELMβ sets the threshold for microbiome-dependent oral tolerance. Nature. 2025 Feb;638(8051):760-768.
- Arnau-Soler A, Tremblay BL, Sun Y, Madore AM, Simard M, Kersten ETG, Ghauri A, Marenholz I, Eiwegger T, Simons E, Chan ES, Nadeau K, Sampath V, Mazer BD, Elliott S, Hampson C, Soller L, Sandford A, Begin P, Hui J, Wilken BF, Gerdts J, Bourkas A, Ellis AK, Vasileva D, Clarke A, Eslami A, Ben-Shoshan M, Martino D, Daley D, Koppelman GH, Laprise C, Lee YA, Asai Y. Food Allergy Genetics and Epigenetics: A Review of Genome-Wide Association Studies. Allergy. 2025 Jan;80(1):106-131.
- Sindher SB, Nadeau KC, Chinthrajah RS, Leflein JG, Bégin P, Ohayon JA, Ponda P, Wambre E, Liu J, Khokhar FA, Akinlade B, Maloney J, Orengo JM, Hamilton JD, Kamal MA, Hooper AT, Patel N, Patel K, Laws E, Mannent LP, Radin AR. Efficacy and Safety of Dupilumab in Children With Peanut Allergy: A Multicenter, Open-Label, Phase II Study. Allergy. 2025 Jan;80(1):227-237.
- Satitsuksanoa P, van de Veen W, Tan G, Lopez JF, Wirz O, Jansen K, Sokolowska M, Mirer D, Globinska A, Boonpiyathad T, Schneider SR, Barletta E, Spits H, Chang I, Babayev H, Tahralı İ, Deniz G, Yücel EÖ, Kıykım A, Boyd SD, Akdis CA, Nadeau K, Akdis M. Allergen-specific B cell responses in oral immunotherapy-induced desensitization, remission, and natural outgrowth in cow’s milk allergy. Allergy. 2025 Jan;80(1):161-180.
- Seastedt H, Han X, Fernandes A, Galli SJ, Boyd SD, Nadeau KC, Manohar M, Chinthrajah S. Association of cytotoxic effector memory CD8+T cells with sustained unresponsiveness after peanut oral immunotherapy. Allergy. 2025 Jan;80(1):319-322.
- Sun N, Ogulur I, Mitamura Y, Yazici D, Pat Y, Bu X, Li M, Zhu X, Babayev H, Ardicli S, Ardicli O, D’Avino P, Kiykim A, Sokolowska M, van de Veen W, Weidmann L, Akdis D, Ozdemir BG, Brüggen MC, Biedermann L, Straumann A, Kreienbühl A, Guttman-Yassky E, Santos AF, Del Giacco S, Traidl-Hoffmann C, Jackson DJ, Wang DY, Lauerma A, Breiteneder H, Zhang L, O’Mahony L, Pfaar O, O’Hehir R, Eiwegger T, Fokkens WJ, Cabanillas B, Ozdemir C, Kistler W, Bayik M, Nadeau KC, Torres MJ, Akdis M, Jutel M, Agache I, Akdis CA. The epithelial barrier theory and its associated diseases. Allergy. 2024 Dec;79(12):3192-3237.
- Zhou X, Simonin EM, Jung YS, Galli SJ, Nadeau KC. Role of allergen immunotherapy and biologics in allergic diseases. Curr Opin Immunol. 2024 Dec;91:102494.
- Marques-Mejias A, Bartha I, Ciaccio CE, Chinthrajah RS, Chan S, Hershey GKK, Hui-Beckman JW, Kost L, Lack G, Layhadi JA, Leung DYM, Marshall HF, Nadeau KC, Radulovic S, Rajcoomar R, Shamji MH, Sindher S, Brough HA. Skin as the target for allergy prevention and treatment. Ann Allergy Asthma Immunol. 2024 Aug;133(2):133-143.
- Nakonechna A, van Bergen A, Anantharachagan A, Arnold D, Johnston N, Nadeau K, Rutkowski K, Sindher SB, Sriaroon P, Thomas I, Vijayadurai P, Wagner A, Davis CM. Fish and shellfish allergy: Presentation and management differences in the UK and US-analysis of 945 patients. J Allergy Clin Immunol Glob. 2024 Jul 22;3(4):100309.
- Hong X, Nadeau K, Wang G, Larman B, Smith KN, Pearson C, Ji H, Frischmeyer-Guerrerio P, Liang L, Hu FB, Wang X. Metabolomic profiles during early childhood and risk of food allergies and asthma in multiethnic children from a prospective birth cohort. J Allergy Clin Immunol. 2024 Jul;154(1):168-178.
- Barshow S, Tirumalasetty J, Sampath V, Zhou X, Seastedt H, Schuetz J, Nadeau K. The Immunobiology and Treatment of Food Allergy. Annu Rev Immunol. 2024 Jun;42(1):401-425.
- Tang SKY, Castaño N, Nadeau KC, Galli SJ. Can artificial intelligence (AI) replace oral food challenge? J Allergy Clin Immunol. 2024 Mar;153(3):666-668.
- Voskamp AL, Khosa S, Phan T, DeBerg HA, Bingham J, Hew M, Smith W, Abramovitch J, Rolland JM, Moyle M, Nadeau KC, Lack G, Larché M, Wambre E, O’Hehir RE, Hickey P, Prickett SR. Phase 1 trial supports safety and mechanism of action of peptide immunotherapy for peanut allergy. Allergy. 2024 Feb;79(2):485-498.
- Castaño N, Chua K, Kaushik A, Kim S, Cordts SC, Nafarzadegan CD, Hofmann GH, Seastedt H, Schuetz JP, Dunham D, Parsons ES, Tsai M, Cao S, Desai M, Sindher SB, Chinthrajah RS, Galli SJ, Nadeau KC, Tang SKY. Combining avidin with CD63 improves basophil activation test accuracy in classifying peanut allergy. Allergy. 2024 Feb;79(2):445-455.
- Croote D, Wong JJW, Pecalvel C, Leveque E, Casanovas N, Kamphuis JBJ, Creeks P, Romero J, Sohail S, Bedinger D, Nadeau KC, Chinthrajah RS, Reber LL, Lowman HB. Widespread monoclonal IgE antibody convergence to an immunodominant, proanaphylactic Ara h 2 epitope in peanut allergy. J Allergy Clin Immunol. 2024 Jan;153(1):182-192.e7.
- Balz K, Kaushik A, Cemic F, Sampath V, Heger V, Renz H, Nadeau K, Skevaki C. Cross-reactive MHC class I T cell epitopes may dictate heterologous immune responses between respiratory viruses and food allergens. Sci Rep. 2023 Sep 8;13(1):14874.
- Lee AS, Parsons ES, Chang I, Dunham D, Chinthrajah RS, Nadeau KC. Quantitative analysis of urinary cytokines in food-allergic and healthy individuals. Allergy. 2023 Sep;78(9):2523-2526.
- Tirumalasetty J, Barshow S, Kost L, Morales L, Sharma R, Lazarte C, Nadeau KC. Peanut allergy: risk factors, immune mechanisms, and best practices for oral immunotherapy success. Expert Rev Clin Immunol. 2023 Jul-Dec;19(7):785-795.
- Ciliberti A, Zaslavsky J, Morcott T, Bozen A, Samady W, Lombard L, Nimmagadda S, Nadeau K, Gupta R, Tobin M. Evaluating the Food Allergy Passport: A Novel Food Allergy Clinical Support Tool. J Allergy Clin Immunol Pract. 2023 Apr;11(4):1162-1168.e7.
- Sindher SB, Barshow S, Tirumalasetty J, Arasi S, Atkins D, Bauer M, Bégin P, Collins MH, Deschildre A, Doyle AD, Fiocchi A, Furuta GT, Garcia-Lloret M, Mennini M, Rothenberg ME, Spergel JM, Wang J, Wood RA, Wright BL, Zuberbier T, Chin AR, Long A, Nadeau KC, Chinthrajah RS. The role of biologics in pediatric food allergy and eosinophilic gastrointestinal disorders. J Allergy Clin Immunol. 2023 Mar;151(3):595-606.
- Sindher SB, Chin AR, Aghaeepour N, Prince L, Maecker H, Shaw GM, Stevenson DK, Nadeau KC, Snyder M, Khatri P, Boyd SD, Winn VD, Angst MS, Chinthrajah RS. Advances and potential of omics studies for understanding the development of food allergy. Front Allergy. 2023 Mar 24;4:1149008.
- Stankovich GA, Warren CM, Gupta R, Sindher SB, Chinthrajah RS, Nadeau KC. Food allergy risks and dining industry – an assessment and a path forward. Front Allergy. 2023 Mar 29;4:1060932.
- Akdis CA, Akdis M, Boyd SD, Sampath V, Galli SJ, Nadeau KC. Allergy: Mechanistic insights into new methods of prevention and therapy. Sci Transl Med. 2023 Jan 18;15(679):eadd2563.
- Liu EG, Zhang B, Martin V, Anthonypillai J, Kraft M, Grishin A, Grishina G, Catanzaro JR, Chinthrajah S, Sindher T, Manohar M, Quake AZ, Nadeau K, Burks AW, Kim EH, Kulis MD, Henning AK, Jones SM, Leung DYM, Sicherer SH, Wood RA, Yuan Q, Shreffler W, Sampson H, Shabanova V, Eisenbarth SC. Food-specific immunoglobulin A does not correlate with natural tolerance to peanut or egg allergens. Sci Transl Med. 2022 Nov 16;14(671):eabq0599.
- Zhou X, Yu W, Dunham DM, Schuetz JP, Blish CA, DeKruyff RH, Nadeau KC. Cytometric analysis reveals an association between allergen-responsive natural killer cells and human peanut allergy. J Clin Invest. 2022 Oct 17;132(20):e157962.
- Sindher SB, Long A, Chin AR, Hy A, Sampath V, Nadeau KC, Chinthrajah RS. Food allergy, mechanisms, diagnosis and treatment: Innovation through a multi-targeted approach. Allergy. 2022 Oct;77(10):2937-2948.
- Warren C, Sherr J, Sindher S, Nadeau KC, Casale TB, Ward D, Gupta R, Chinthrajah RS. The impact of COVID-19 on a national sample of US adults with food allergy. J Allergy Clin Immunol Pract. 2022 Oct;10(10):2744-2747.


