[ Fall 2012 ]
HSPH’s Will Mair hopes his work in worms will identify molecules that have an effect on aging-related diseases—and which could ultimately be tested as treatments for humans.
“How old you are is immutable—you can’t change how old an animal is,” says William Mair, assistant professor of genetics and complex diseases at HSPH. “But you can change how it ages.”
That observation points to a new way of thinking about aging: not as a preordained decline, but as a malleable function of the body. And viewed in this way, aging belongs at the center of public health research. Rather than just treating endpoints—such as cardiovascular disease, metabolic disorders, cancer, and neurodegeneration—could researchers improve population health by targeting the aging process itself?
Mair’s young lab, launched last November, is trying to answer that question. “It’s not enough to say it’s inevitable that we get more frail,” says Mair. “There’s something that happens that makes an old animal more susceptible to getting these disease states. For example, if you look at cancer, one of the most common age-related diseases, it’s clearly not one pathology. Similar tumors can result from very different mutations in different individuals. Trying to find those specific mutations is one way to do research. But if you could make the environment more resistant to developing tumors in the first place, you can try to reduce the chances of getting cancer with age.”
Mair first became intrigued with aging as an evolutionary question: If infirmity isn’t just a product of wear and tear, why do we age at all? His research began with an observation known since the 1930s: A diet severely restricted in calories (about 30 percent below normal, but above starvation levels) can increase lifespan, lower rates of cancer, and slow declines in memory and movement. This effect, first seen in laboratory rats, has been replicated in species as diverse as yeast, fruit flies, worms, and even rhesus monkeys. Further research has uncovered genetic mutations in animals that can mimic the effects of dietary restriction, and some of these same mutations are found in people who live into their 90s.
But laboratory-manipulated longevity also comes with a price. Restricted-diet animals grow more slowly, reproduce less, and have dampened immune systems. More than just cutting calories, dietary restriction seems to switch the body into a survival mode in which growth and energy consumption are suppressed.
Today, stalwart human volunteers are testing whether dietary restriction works in humans, both on their own and as part of studies like the ongoing federally funded clinical CALERIE trial (for Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy). “It’s not something I would advocate doing,” Mair says, not only because food deprivation is unpleasant, but also because it could produce similar negative side effects in humans, such as fertility problems or susceptibility to infections.
Mair wants to see if there’s a way to tap into the health benefits of dietary restriction without the negative side effects. “We want to try to uncouple the good from the bad,” he says. “And to do that, you need a system that you can play around with genetically.”
A Fast-forward view of aging
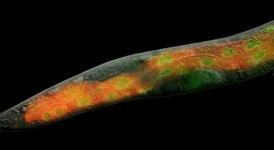
His subject of choice: Caenorhabditis elegans, the classic laboratory nematode used across a wide field of research. These tiny, transparent worms have played a central role in aging research. Though just a millimeter long and composed of barely a thousand cells, they show visible signs of aging: they slow down, stop reproducing, and even develop wrinkled skin. Easy to manipulate genetically, and with a life span of just two weeks, C. elegans provides a quick time-lapse view of the aging process. That speed suits Mair, whose rapid speech bears inflections from his native Suffolk, England.
“What on earth can we learn about humans from studying a worm? One answer is that the worm is a way to investigate causality. You can learn a lot of stuff by doing an epidemiological study to find out what’s changing in population—but it’s very hard to find causality in those changes. With this simple worm, in a cheap and quick way, we can tweak things and find causality. And if we do that, coupled with what we know from colleagues who are working on how these genes are linked to different pathologies, then it can be a very powerful model.”
Joining Forces: Aging and Disease
After completing a postdoctoral fellowship at the Salk Institute for Biological Studies in La Jolla, California, Mair moved to the School to collaborate with scientists studying the chronic diseases he believes his research can help alleviate. There’s reason to join forces: Many of the same genes and cellular processes involved in aging also play a role in diabetes, obesity, and cancer. Mair, for instance, recently received an award for a pilot project through HSPH’s Transdisciplinary Research on Energetics and Cancer (TREC), a program funded by the National Cancer Institute to promote research on links between obesity and cancer.
“I’m not looking at cancer or obesity in the worm,” he says. “I’m looking at energy sensing and how that affects stress resistance and healthy aging. But it involves exactly the same molecules.” For example, some of the patients taking the widely prescribed diabetes drug metformin appear to be resistant to certain cancers—an outcome unrelated to the protective effect that the drug has on diabetes. Mair’s lab has shown that activating one of the key molecular targets of metformin in worms makes them age more slowly. Seeing the same disease pathways turn up in research across widely separated disciplines argues for a more integrated research approach.
Mair hopes that his work in the worm will identify molecules that have an effect on aging-related disease, which could then be tested in mice and eventually in humans as possible therapies. But for now, he’s focused on making basic discoveries rather than hunting for drugs. He sets himself apart from scientists who explicitly want to boost lifespan in humans, which he says has given studies on aging a Frankenstein-like reputation among the public.
“You have to walk a fine line in the field,” he says. “There are certainly members of it who don’t. Their motivation is that they want a pill to make themselves live a long time,” he says. “Some people—and it’s a very small minority who are not well-credited in the aging field—have said that the first human to live to 500 is alive right now. There’s no scientific basis for that. It’s so detached from my reason for working on these questions, it’s sci-fi rather than natural science. We have a long way to go.”
Fresh Perspectives, Ethical Questions
At the same time, tinkering with the aging process could have huge public health repercussions. “Everybody knows someone who’s had cancer or type 2 diabetes or Alzheimer’s disease. They see how it destroys people’s lives and they’re scared of it,” says Mair. As for the argument that research on prolonging a healthy life is unethical, because the planet is already too crowded: Mair doesn’t buy it. “Everything that alleviates suffering is unethical not to do,” he says. “All public health strategies, if successful, will help more people survive to older ages—and hopefully, succumb less to chronic diseases. How we cope as a species with the effect that might have on the age structure of our population is a separate issue.”
Though he explores the fundamentals of aging, Mair, who is 33, cultivates a decidedly fresh presence on his HSPH website. One link features a ticking digital clock showing the exact age of the lab, down to the second. Another tracks the music playing in the lab (from Esperanza Spalding to Sigur Rós to Radiohead). The lab also posts Twitter messages.
“Being a young lab is a difficult thing. You’re trying to get things off the ground,” says Mair. “The ticking clock is meant to reflect a certain level of honesty about how long we’ve been here. The more transparency you have—showing what’s going on, that we’re progressing and moving—the better. We want people to feel excited about our work. It’s also very important to make a lab a community. If you can give the lab a personality, it helps you recruit. This lab website has a face—it’s not just an entry on the faculty page.”
Courtney Humphries is a Boston-based science journalist and author.
Learn more
TREC Center at HSPH Tackles Obesity, Cancer Prevention (HSPH News)
